 Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial
Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial

Create a Profile

Create a Profile

Get In Touch Mobile
Date Published: 06/21/2022
Accel principal investigator shares thoughts on COVID vaccines for young kids
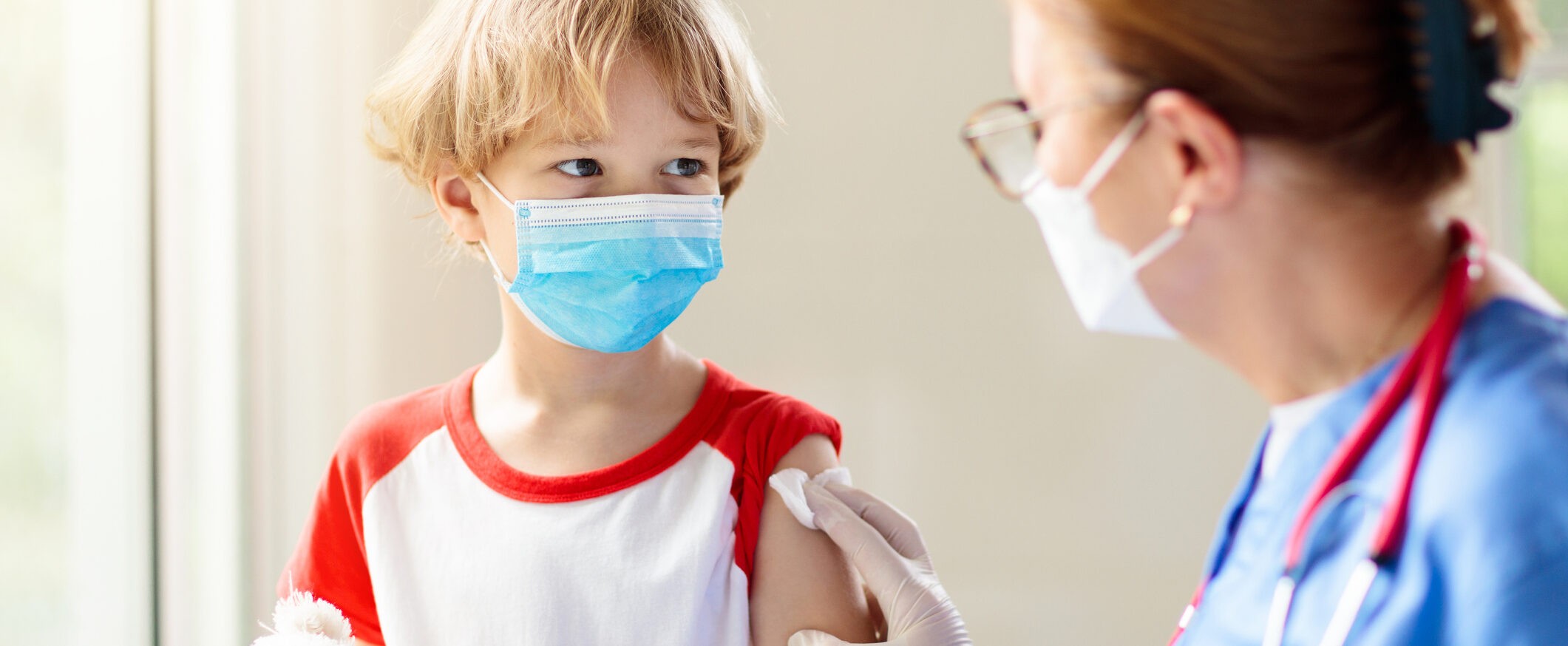
The CDC recently approved Pfizer and Moderna’s COVID-19 vaccines for children as young as 6 months.
Dr. Salma Elfaki, a pediatrician who worked on pediatric vaccine clinical trials with Accel Research Sites, recently spoke with WKMG in Orlando as the first shipment of the COVID-19 vaccines for children were set to arrive at her office. She shared more about why this approval is important.
Read more here.
Articles
 Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial
Accel Research Sites named top enroller for cutting-edge Alzheimer’s disease clinical trial
 Principal investigator discusses increase in overdoses associated with fentanyl
Principal investigator discusses increase in overdoses associated with fentanyl
 Tampa TV station shares stories of participants in Alzheimer’s trial
Tampa TV station shares stories of participants in Alzheimer’s trial
Ready to be part of healthcare history? Find the right clinical trial for you.


